Diatomaceous earth
Diatomaceous earth, also known as diatomite, is a naturally occurring silicate fine stone that easily breaks down into a fine white/off-white powder. This powder contains particles of sizes smaller than 1 micron to more than 1 mm, but usually 10 to 200 microns. This powder has an abrasive effect similar to mica powder and is very light due to its highly porous nature. Typical chemical composition of dried diatomaceous earth is 80 to 90% silicon oxide, with 2 to 4% aluminum oxide (which mainly causes clay mineral content) and 0.5 to 2% iron oxide.
Diatomaceous earth consists of fossil remains of diatoms-algae characteristic by hard-coated shell. It is used as a filtering agent such as a fine abrasive a mechanical insecticide, an absorbent of liquids, such as litter for cats, as an activator of blood coagulation and as a stabilizing component of dynamite. Because of its resistance to temperature, it can be used as a thermal insulator.
Formation
Diatomite is formed by the accumulation of amorphous silica (opal, SiO2 · nH2O) - remnants of dead diatoms (microscopic unicellular algae) in lake and marine sediments. Fossil remains consist of a symmetrical shells or shells.
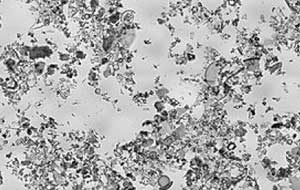
Microscopic view of diatomaceous earth under bright incandescent light
Diatomaceous earth was formed from shells of unicellular diatoms which easily disintegrate into fine powder. The walls of diatoms consist of shells of biogenetic silica synthetized into diatom cells by polymerization of silicic acid. This image shows suspensions of diatomaceous earth particles in water in range of 6,236 pixels in a layer about 1.13 to 0.69mm.
Thermal efficiency
Thermal properties enable this material to be used as a protective material for various firefighting purposes. It can also be used as a vacuum powder insulation in cryotechnology. Diatomaceous earth powder are brought into the vacuum space as auxiliary material for effective vacuum insulation.
back to top